High Performance Liquid Chromatography
Introduction
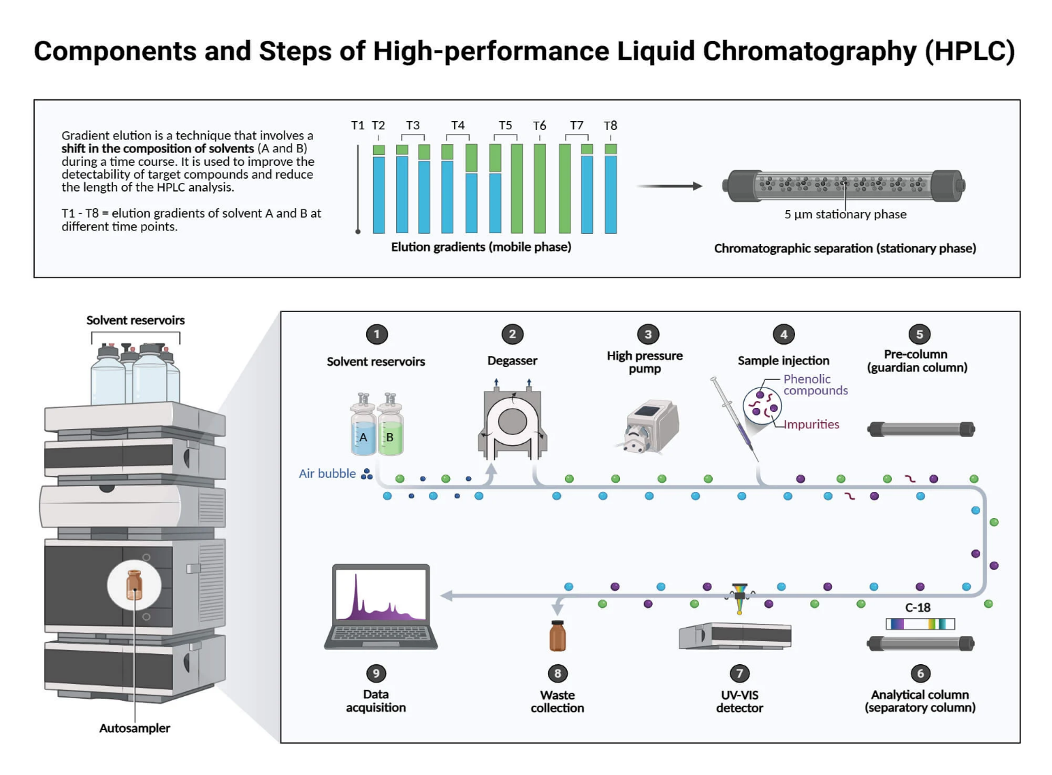
High Performance Liquid Chromatography (HPLC) is a technique for separating analytes dissolved in a mobile phase by using their specific interaction with a stationary phase to identify and quantify each component in the mixture. The mixture is separated using the basic principle of column chromatography and then identified and quantified via spectroscopy.
In case of HPLC, a microgram amount of the sample is allowed to pass through a column containing a stationary solid inert phase coated with a non-volatile phase by means of a 400 atmosphere pressured insert of gravitational force flow of a liquid mobile phase where components migrate at a much faster and at different rates. This is the most advantageous feature which makes it a versatile technique; however, one must choose the mode for a given sample.
Principle
HPLC components of interest are separated based on their physiochemical interaction with the stationary and mobile phase. A high-pressure pump drives the mobile phase via the resolving power of a chromatographic column where column length and number of theoretical plates per unit length matter. The solvent component is then separated based on polarity, size, or charge, which are partially different. Later, the separated particle is detected by detectors such as UV-Vis, fluorescence, or mass spectrometry to identify and quantify the separated compounds.
HPLC Instrument
- Solvent Reservoir: Mobile phase contents are stored in a glass reservoir. In HPLC, usually a mixture of polar and non-polar liquid constituents is used as the mobile phase, or solvent, where the concentration of constituents varies based on the composition of the sample.
- Pump: It is the heart of the instrument and determines the high resolution and the speed of solution. It sucks the mobile phase from the solvent reservoir and pushes it through the column and detector. Operating pressures of up to 42000 kPa (about 6000 psi) can be generated based on factors that include the dimension of the column, the particle size of the stationary phase, the composition of the mobile phase, and its flow rate.
- Sample Injector: The sample solution is usually introduced by a micro syringe into the head of the column. In the HPLC system, the injection of the sample is facilitated by the injector, where the liquid sample is within the range of 0.1-100 mL of volume with increased reproducibility and pressures up to 4000 psi.
- Column: Columns are typically made up of stainless steel, glass, aluminum, or copper. Their length ranges between 50 and 300 mm and the internal diameter between 2 and 5 mm. They are packed with stationary phase particles of size 3–10 µm. Columns are packed with solids like silica or alumina; these columns are called homogeneous columns. If the stationary phase in the column is a liquid, the column is deemed a bonded column, where a liquid stationary phase is bonded to a solid support, generally made up of alumina or silica.
- Detector: Since the quantity of material applied to the column is very small, it is imperative that the sensitivity of the detector system is high. Commonly used detectors are UV detector, fluorescence detector, mass-spectrometer, and electrochemical detectors.
- Data Collection Devices: Chart detectors and electronic integrators are used to collect the signals from the detector. These chart recorders and electronic integrators vary in their intricacy and in the ability to process, store, and then process the chromatographic data.
- Retention Time: The total time taken by the compound to travel to the detector through the column is called retention time. This time is initiated when the sample is injected until the point at which the display shows a maximum peak height for the compound being run. Different compounds show diverse retention times, which vary depending on factors such as pressure applied, nature of the stationary phase, solvent composition, and column temperature.
Types of HPLC
There are several variants of HPLC depending upon the phase system in the process:
- Normal Phase: This separates the analytes on the basis of polarity. It uses a polar stationary phase (e.g., silica) and a non-polar mobile phase.
- Reverse Phase: In this case, the stationary phase is non-polar (e.g., C18) while the mobile phase consists of polar liquids such as a mixture of water and methanol. This works on the principle of hydrophobic interaction.
- Size Exclusion: Here, the column contains material with precisely controlled pore size, and the particles are separated according to their molecular size.
- Ion Exchange: The stationary phase has an ionically charged surface of opposite charge to the sample ion. The stronger the charge on the sample, the stronger it will be attracted to the ionic surface and, therefore, takes longer to elute.
Working of HPLC
Figures illustrating the working of HPLC instruments and the flow of the process are referenced but not included here. For visual representation, refer to external sources for diagrams of HPLC workflows.
Applications of HPLC
HPLC has become universally applicable and addresses diverse forensic needs:
- Toxicology: HPLC-MS helps detect poisons (e.g., cyanide, pesticides) as well as drugs (e.g., opioids, cocaine) in blood, urine, or hair.
- Drug Analysis: It also quantifies illicit substances in seized material and helps distinguish isomers. Reverse phase HPLC is standard for methamphetamine purity testing.
- Trace Evidence: HPLC helps analyze dyes, ink, and even explosive residues, aiding in linking suspects to crime scenes.
- Environmental Analysis: It aids in pollutant identification (e.g., herbicides) in poisoning cases.
- Post-Mortem Analysis: It helps determine the time of death by measuring vitreous humor metabolites, using ion-exchange HPLC to detect amino acid degradation.
Advantages and Disadvantages of HPLC
| Advantages | Disadvantages |
|---|---|
| High Sensitivity | High Cost |
| Versatility | Complex Sample Preparation |
| High Resolution | Interference Risk |
| Quantitative Accuracy | Specialized Expertise |
| Non-Destructive | Time Intensive |
HPLC is a vital tool in forensic science for precise analysis of drugs, toxins, and trace evidence. Further advancements like HPLC-MS/MS and UHPLC have accelerated investigations while providing more efficiency.
References
- What is High-Performance Liquid Chromatography (HPLC)?. Agilent.
- Aryal, S. (2025, June 14). HPLC: Principle, Parts, Types, Uses, Diagram. Microbe Notes.
- HPLC Basics. Thermo Fisher Scientific.